28Aug 2022
GN Corporation has published a paper on its beta glucan product neu REFIX in the Journal of Clinical and Experimental Hepatology.
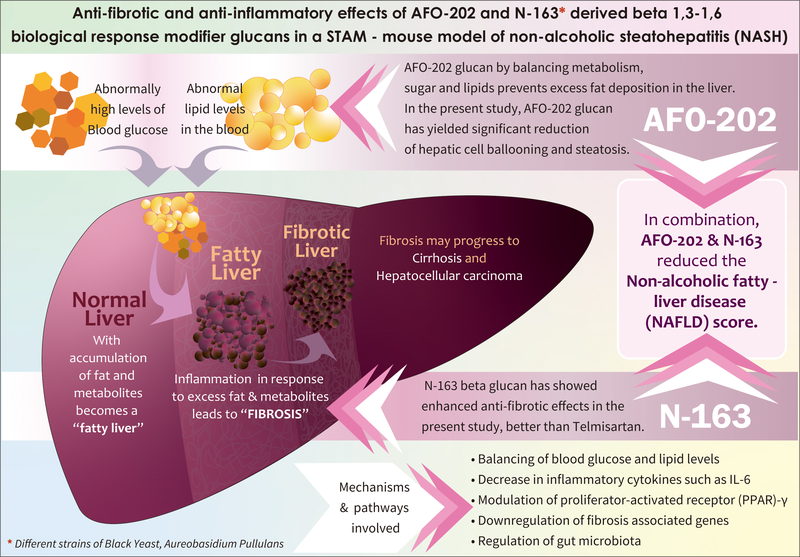
The paper, “Hepatoprotective effects of beta-1,3-1,6-glucan from black yeast in a mouse model of non-alcoholic fatty liver” (https://doi.org/10.1016/j.jceh.2022.06.008), reported that beta-glucan showed beneficial results against a disease called non-alcoholic fatty liver (NASH) in the animal study.
Now that neu REFIX has been shown to have an immunomodulatory function and control inflammation and fibrosis in NASH, a new short-term clinical trial is underway for Duchenne muscular dystrophy, a rare disease caused by a genetic mutation that has similar pathophysiology. The study will focus on inflammation and fibrosis biomarkers, similar to the study in NASH (preprint article: https://doi.org/10.1101/2021.12.13.21267706 ).
The Duchenne Muscular Dystrophy Study Group Duchenne Awareness Day will be held on September 4, 2012. Dr. Yoshitsugu Aoki, Director of Gene Therapy Research, National Center of Neurology and Psychiatry (NCNP), will give a special lecture, and Dr. Raghavan of Department of Pediatric Neurology, JAICARE, Tamil Nadu, India, will present a clinical trial of neu REFIX in India.
Please see Kyodo News PR Wire press release.